Isotopes and Elements - the Atomic Nucleus
A simple atom such as hydrogen has a single proton as its atomic nucleus around which a single electron orbits. The proton (p+) is positively charged and the electron (e-) is negatively charged. If we assign the proton a mass of 1, the electron will have a mass of 0.0005, and thus is 2000 times lighter. A visual comparison might be that of a feather that is blown across the ground by light winds. Analogously, the feather-light electron is constantly moving about the atomic nucleus, and does not form part of the nucleus.
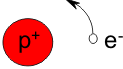
Another building block of the atomic nucleus is the neutron. To explain its function and the forces that occur in the atomic nucleus, we will digress briefly to describe an example familiar to everyone: magnetism.
With two permanent magnets, there is an attractive force (F) between the north pole and south pole, but a repulsion is experienced when two north poles or two south poles are held together.
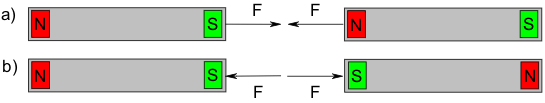
This force acts even when the magnets are still far apart. The situation is different if a piece of iron is brought in the vicinity of the magnets. Magnetism develops only once the iron is close to the magnet, because then the atoms inside the piece of metal become reoriented due to the magnetic field.
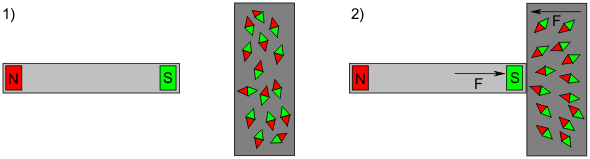
Comparing these examples, one of them demonstrates a permanent force that acts over long distances while the other force only arises in close proximity.
Returning to the atom, a similar force act between protons and electrons. This is “electrostatic force”, a permanent attraction between oppositely-charged objects that acts over a long distance. While an electron will stay in its orbit due to attraction by the proton, two positively-charged protons will repel each other.
![]()
The question arises of how atomic nuclei that contain multiple protons can exist - orbited by multiple electrons - as they are known to do in dozens of other chemical elements beyond hydrogen, such as oxygen, nitrogen and carbon.
For one thing, there is another nuclear constituent (or particle) with neutral charge that acts like the mortar holding the building blocks of the atomic nucleus together - this is the neutron (n), with a mass of 1. For another, both protons and neutrons bind to each other on contact like sticky pieces of warm taffy. Just as the magnetization of the piece of iron depends on the orientation of the iron atoms, even smaller building blocks within the protons and neutrons become oriented with respect to each other - these smaller building blocks are quarks. The forces between the nuclear particles are called nuclear forces (shown in blue). For protons, the attraction due to the nuclear force is stronger than the repulsive force due to their electric charges:
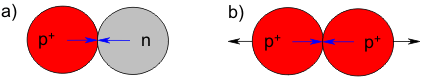
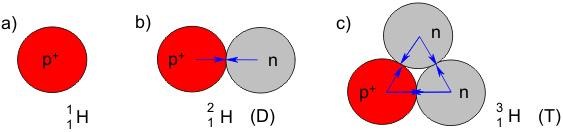
Incidentally, the structure of an atomic nucleus can be indicated with the following symbolic format (H is the element symbol for hydrogen):
![]()
The lower number indicates the number of protons, while the upper number indicates the sum of all the nuclear particles. This means that there is only one proton present in hydrogen.
Even though most hydrogen atoms consist of one proton and one electron, hydrogen atoms with neutrons also exist, such as deuterium (D) and tritium (T). Atoms that differ only in the number of neutrons present are called isotopes. The hydrogen isotopes are thus “light hydrogen”, deuterium (also called heavy hydrogen) and tritium. Deuterium makes up 0.015% of all hydrogen atoms, for example, when bonded in the compound water (H2O) as "semi-heavy water" HDO and “heavy water” D2O. In contrast to the relatively stable deuterium, tritium occurs only rarely in nature, and decays with the release of radiation.
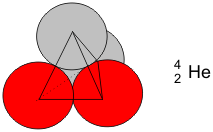
Increasing the number of protons leads to different chemical elements. Hydrogen contains one proton, helium two, beryllium three, lithium four, boron five, carbon six, and so forth. These chemical elements can all occur as isotopes, which vary in their stability. For example, the atomic nucleus of helium (He) composed of two protons and two neutrons is particularly stable. The nuclear particles are arranged in a tetrahedron, in which all four of them are bound by nuclear forces. The repulsive electrostatic forces between the protons are negligible in comparison to the nuclear forces.
In the case of carbon, for example, C-12, C-13, and C-14 occur in nature (in this shorthand, the total number of nuclear particles follows the atom symbol with a hyphen between). C-12 thus contains six protons and six neutrons and makes up 98.9% of all carbon, while C-13 thus contains six protons and seven neutrons and occurs to the extent of 1.1%. C-14 is made up of six protons and eight neutrons; it occurs only in traces and undergoes radioactive decay.
Why are some isotopes stable and other unstable? We recall the situation with the magnets, and that the electrostatic repulsive forces of protons operate over longer distances, but the attractive nuclear forces only operate directly between the nuclear particles at short range. The more protons that are present, the larger will be the sum total of repulsive electrostatic forces.
A plot of the number of protons (P) vs. the number of neutrons (N) gives a graph of the known isotopes. It is obvious that the optimal ratio of neutrons to protons begins as approximately 1:1 for various chemical elements, while the ratio then tends toward 1.5:1. Thus, the larger the atom, the larger the number of neutrons necessary to balance the electrostatic interactions. The stable nuclei are shown in the graph as black points, while those shown in other colors are the unstable ones that can undergo decay:
Thus there are some atoms that are unstable because the number of neutrons is too low, while there are also those that undergo decay because the number of neutrons is too high.
As a rule, very large nuclei (starting from element 83) are unstable, and there are also unsuitable ratios of neutrons to protons that lead to nuclear decay. One can understand best from the following picture: a compact snowball is stable and can exist over longer periods of time. On the other hand, a large slab of snow can collapse spontaneously and cause an avalanche.
In fact, the exact explanation of the interactions within the atomic nucleus is a topic of current research. One interesting question, for example, is whether there are very heavy elements, totally unknown up to the present time, that exist in an “island of stability” at higher numbers of nuclear particles.
Some unstable isotopes (some that decay in a fraction of a second, or after thousands of years) were formed when the universe was still very young and hot. They can also be formed through nuclear fusion (the “merging” of two atomic nuclei) in the Sun at high temperatures, or artificially when a nucleus undergoes bombardment by neutron, such as in a nuclear power plant. An example is tritium, which is carried to Earth in the solar wind, and is also formed in the upper layers of our atmosphere.
